Boiler Water Chemistry
THE REASON FOR CHEMICAL WATER TREATMENT
PART 1 - How can WATER hurt your boiler?
Boiler corrosion is the active destruction of solid boiler metal by the pitting action of dissolved oxygen in the boiler water. This usually results in deep holes in the metal which are self-protected by further corrosion products i.e. scabs or blisters over the hole which allow the chemical reaction to continue.

Corrosion can be caused by oxygen attack, acidic reaction at the corrosion cell or under deposit reaction (under scale corrosion). Oxygen corrosion takes the form of localized deep pitting and can quickly lead to tube failure. Oxygen attack can cause further damage to steam drums, mud drums, boiler headers, and condensate piping.
Oxygen Removal
The addition of oxygen to a system with feed water and heat will initiate corrosion.
- Heat accelerates the oxygen corrosion if the oxygen is not removed from the feedwater system and boiler water
- An oxygen scavenger is used to eliminate the remaining oxygen.
- Properly applied, oxygen scavengers will prevent oxygen pitting
- The ability for oxygen to attack forming a pit on metal, increases by a factor of two for every 10ºC) rise in temperature.
- Oxygen can be 512 times as aggressive at 100º than it was at 10ºC.
- Once heated, the oxygen has to be removed via mechanical and chemical means.
- Oxygen pitting occurs when the oxygen has been heated up and then is not removed.
Oxygen Scavengers
Heating the feed water greatly reduces the dissolved oxygen but does not remove all dissolved oxygen
Oxygen scavengers when applied properly, should be able to remove oxygen down to 1-3 ppb by volume
- The Law of Mass Action: Excess feed of a reactant is used to drive a reaction to completion. Therefore, we maintain chemical residuals as Tannin to force the reaction to completion
- Oxygen’s ability to react with iron doubles every 10ºC increase in feedwater temperature.
Oxygen Protection
Tannins – Functions as a filming agent on metal surfaces and also functions as an oxygen scavenger.
- Functional group is a reaction with the free iron to form a complex on the metal surface. Absorption followed by magnetite formation
- Tannins are long chain aliphatic polyanions, which gives them dispersant properties as well as adsorption of hardness ions.
Tannin
Advantages:
- Tannin’s don’t add to the boiler TDS so reduces the need for blow down and saves energy
- Feed water tannins have a dual corrosion protection mechanism since they not only remove the oxygen but also form a corrosion resistant tannate film on the boiler steel
- Tannin is brown in colour so is easier to detect and test for
- They are particularly suitable for low or variable feed water temperatures and very good at protecting idle and intermittently used boilers.
- Suitable for low pressure boilers
- Tannin residual level in the Boiler for Integra Chemical is 120-160ppm
PART 2 - How can WATER hurt your boiler?
Boiler Scale
Boiler scale is a deposit that forms directly on heat transfer surfaces when constituent solubility limits are exceeded and the resulting compounds precipitate onto the tube surfaces. Such deposits may contain sodium, calcium, magnesium, phosphate, iron, and silica.

The disadvantages of scale formation:
Scale has low thermal conductivity so the scale of heat transfer from the boiler to inside water is greatly reduced. In order to provide a uniform supply of heat to the water, additional fuel is consumed to achieve the same temperature.
The chart below shows the fuel loss in a boiler based on thickness of scale:
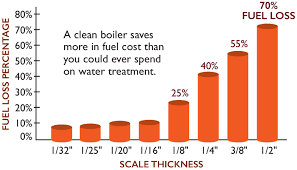
• 1/32” = 8.5%
• 1/25” = 9.3%
• 1/20” = 11.1%
• 1/16” = 12.4%
• 1/8” = 25%
• 1/4” = 40%
• 3/8” = 55%
• 1/2” = 70%
• The economics show that a clean boiler will save far more money in fuel than it could ever use in water treatment.
Feed Water pH/Temperature/Hardness
- The pH of the feed water should be above 8.5 to prevent corrosion of iron in the system
- Optimum pH in the feed water should be 8.5-9.5
- The higher the feed water temperature the lower the dissolved oxygen will be in the feed water. The lower the dissolved oxygen, the lower the potential for corrosion to take place in the boiler water systems
- The feed water needs to be softened water. Water softeners use and ion exchange process to exchange calcium and magnesium hardness with sodium/salt. In the event of scaling in the system the sodium ions are easily removed Vs calcium/magnesium ions.
Cycles of Concentration
Cycles refer to the concentration of solids in the boiler water.
As the water is evaporated, the solids stay behind. These solids are now more concentrated or cycled up
The quality of the Feed water determines how many cycles we can run in the boiler
COC - Boiler TDS / Feed water makeup - 1 = COC
Example 1 – 3000 TDS / 800 TDS – 1 = 2.75 COC
Example 2 – 3000 TDS / 400 TDS – 1 = 6.50 COC
Total Dissolved Solids (TDS)
- Over time the level of total dissolved solids(TDS) increases.
- Further evaporation causes these dissolved solids to come out of solution, and to produce suspended solids (sludge).
- TDS is measured as both Neutralised and un – neutralised.
Controlling Total Dissolved Solids in the boiler water
As a boiler generates steam, any impurities which are in the boiler feed water and which do not boil off with the steam will concentrate in the boiler water.
As the dissolved solids become more and more concentrated, the steam bubbles tend to become more stable, failing to burst as they reach the water surface of the boiler. There comes a point (depending on boiler pressure, size, and steam load) where a substantial part of the steam space in the boiler becomes filled with bubbles and foam is carried over into the steam main.
This is obviously undesirable not only because the steam is excessively wet as it leaves the boiler, but it contains boiler water with a high level of dissolved and perhaps suspended solids. These solids will contaminate control valves, heat exchangers and steam traps.
Whilst foaming can be caused by high levels of suspended solids, high alkalinity or contamination by oils and fats, the most common cause of carryover (provided these other factors are properly controlled) is a high Total Dissolved Solids (TDS) level. Careful control of boiler water TDS level together with attention to these other factors should ensure that the risks of foaming and carryover are minimised.
Neutralised total dissolved solids
Neutralised total dissolved solids are measured by adjusting the pH of the sample water to 7 with a neutralising reagent and then testing with a TDS meter.
This give the corrected TDS reading and is normally lower by a third than the TDS tested without Neutralisation.
It is done to neutralize the hydroxyl ions (OH- ). Hydroxyl ions contribute significantly to the total ionic concentration of the water sample. This affects both the conductivity and TDS readings when based on the specific conductance of the sample.
Why do we want to neutralize the effect of hydroxyl ions on our readings? Hydroxyl ions are not considered a dissolved solid, but instead part of a disassociated water molecule. When we take our conductivity/TDS readings on the boiler water and calculate cycles of concentration, we want to have a truer estimate of the cycles of concentration of dissolved solids without the added conductance effects of hydroxyl ions.
Caustic Alkalinity (Hydroxide Alkalinity)
Caustic Alkalinity in steam boilers is very important in minimizing the potential or scale formation
Boiler water must contain a certain amount of causticity i.e. Hydroxide alkalinity in order to accomplish three things:
1. To maintain a protective coating of iron oxide over the metal and thereby prevent certain types of corrosion.
2. To provide a proper environment for precipitation of desirable sludge materials.
3. To keep silica in solution so as to avoid deposition of silica scales.
Example of ideal steam boiler operating parameters:
Un neutralised TDS Minimum 2500 , Maximum 3000
% of Caustic Alkalinity 10 to 30 %
Tannin Levels 120 to 160 ppm or 12-16 tannin index
Automatic blowdown devices to be calibrated and adjusted when conducting TDS testing
Manual blowdowns to be conducted once a day to blowdown and remove dirt and solids from the mud drum, best practice to conduct at the start of the day.
For more information and advice on boilers and boiler water chemistry, contact Integra today.









